What Is TB-500 5MG?
TB-500 is a synthetic derivative of thymosin beta-4 (T????4). T????4 is a naturally occurring peptide composed of 43 amino acids. It is produced by the thymus gland and is found in body fluids, including saliva, blood, tears and wound fluid. Research shows that T????4 regulates various biological processes, such as cell differentiation, wound healing and tissue regeneration. The existing literature mentions research on T????4 only. As the structural analog of T????4, TB-500 is expected to replicate similar results.
Structure Of TB-500 5MG
From Pubchem
Synonyms: QHK6Z47GTG, TB-500
Molecular Formula: C38H68N10O14
Molecular Weight: 889.0 g/mol
Sequence: LKKTETQ
CAS number: 885340-08-9
PubChem CID: 62707662
History
Thymosin beta-4 was first isolated from the bovine thymus gland extract by Low and Goldstein in 1981. In the early 2010s, its synthetic analog, TB-500, was developed for veterinary use. Due to its potential performance-enhancing properties, its use became popular in competitive horse racing. Consequently, the World Anti-Doping Agency (WADA) banned its use in sports. At Pinnacle Peptides, Tb-500 for sale is restricted to research and educational purposes, not for human consumption.
Mechanism Of TB-500 5MG
Actin is a globular protein responsible for various cellular processes, including movement, cell shape and mechanical strength. Actin exists in two forms: G-actin and F-actin. G-actin monomers polymerize to form F-actin, which together with other proteins combine to form microfilaments. Thymosin beta 4 is an actin-sequestering molecule. It binds to G-actin and blocks its polymerization. Sequestering actin at the site of injury signals the body to migrate other stem and progenitor cells to the location, thus promoting connective tissue regeneration and healing. T????4 is also thought to reduce the expression of pro-inflammatory cytokines and microRNA-146a (miR-146a), thus suppressing inflammatory response.
Pre-Clinical/Clinical Research
1. Dry Eyes
Research shows that T????4 treatment might prove effective for healing of corneal surface. One study found that 0.1% T????4 therapy relieved symptoms of dry eyes and no adverse effects were reported [1]. In another study, researchers assessed the safety and efficacy of T????4 eye drops in patients with severe dry eyes. The participants were randomized to receive either T????4 eye drops or placebo six times a day for 4 weeks. The results found that thymosin beta 4 reduced ocular discomfort by 35.3%, increased tear production and reduced total corneal fluorescein staining by 59.1% reduction [2].
2. Muscular Dystrophy
Duchenne muscular dystrophy is a rare genetic disorder that affects muscle function and leads to muscle degeneration. A study was conducted on a mouse model of Duchenne muscular dystrophy to find the effectiveness of Thymosin beta 4. The subjects were either treated with T????4 or placebo two times a week for six months. The results indicated the increased number of muscle regenerating fibres with T????4 as compared to the placebo. These findings suggest that Tb-500 has the potential to treat muscular dystrophy [3]. Moreover, research shows that T????4 repairs damage to heart muscles and promotes the survival of cardiomyocytes [4].
3. Angiogenesis
Research shows that TB-500 promotes neovascularization, and the formation of new blood vessels. Research conducted on mice found that Tβ4 is important for angiogenesis. It revealed that thymosin beta-4 stimulates the growth of previously dormant epicardial explants. Further, it promotes differentiation of fibroblasts and endothelial cells [5].
One study was conducted to suggest the possible mechanism involved in the angiogenesis caused by Tβ4. The test containing endothelial cells was treated with thymosin beta 4 along with inhibitors of AKT and PI3Kα/Rho pathway. Plus, Tβ4 was injected along with rAAV.AKT-dn to animal subjects. It is important to note that AKT is important to activate the PI3Kα pathway, while AKT-dn is the altered form of AKt that disturbs its function. The findings indicated that inhibitors of PI3kα/AKT reduced the angiogenic effect of Tβ4, suggesting the involvement of this pathway in mediating angiogenesis [6].
4. Injury Recovery
Multiple animal studies prove that thymosin beta-4 (Tβ4) is beneficial for wound healing and accelerating the recovery process after injuries. One study conducted on rats proved the effectiveness of Tb-4 in treating incisional wounds while minimizing scarring. The wounds treated with Tβ4 were narrower with more organized collagen fibers and displayed red birefringence, a sign of mature connective tissues. On the other hand, untreated wounds showed green birefringence, an indication of immature connective tissues, and disorganized collagen fibers [7].
In another study involving rats, Tβ4 treatment six hours after traumatic brain injury led to a reduction in the size of cortical lesions and an improvement in functional recovery. However, treatment given after 24 hours of injury did not affect the size of cortical lesions but improved functional recovery in rats [8].
Additionally, in a randomized double-blind, placebo-controlled phase II trial with dose escalation, topical application of Tβ4 was found to enhance wound healing in patients with venous ulcers. Plus, the researchers found that a 0.03% dose of synthetic thymosin beta-4 helped 25% of patients with small to moderate-sized wounds achieve full recovery [9].
The mechanism behind this effect involves actin-sequestering properties of thymosin beta-4. At the site of injury, thymosin beta-4 binds to actin, promoting the migration and differentiation of stem cells. This, in turn, leads to the formation of new blood vessels that facilitate the process of wound healing. Furthermore, it reduces the number of myofibroblasts at the injury site, ultimately minimizing scar formation [10].
Summary
Various studies on animal models show that Thymosin beta-4 promotes angiogenesis and wound recovery. However, we need studies in humans to confirm its safety and efficacy. FDA hasn’t approved it yet. Plus, it is banned by WADA and is only available as an experimental drug. We don’t support its unwarranted use and offer TB-500 purchase for research only. Only buy Tb-500 if you are an authorized researcher.
References
- Sosne, G., et al., Thymosin Beta 4 Eye Drops Significantly Improve Signs and Symptoms of Severe Dry Eye in a Physician-Sponsored Phase 2 Clinical Trial. Investigative Ophthalmology & Visual Science, 2013. 54(15): p. 6033-6033.
- Sosne, G. and G.W. Ousler, Thymosin beta 4 ophthalmic solution for dry eye: a randomized, placebo-controlled, Phase II clinical trial conducted using the controlled adverse environment (CAE™) model. Clin Ophthalmol, 2015. 9: p. 877-84.
- Spurney, C.F., et al., Evaluation of skeletal and cardiac muscle function after chronic administration of thymosin beta-4 in the dystrophin deficient mouse. PLoS One, 2010. 5(1): p. e8976.
- Bjørklund, G., M. Dadar, J. Aaseth, and S. Chirumbolo, Thymosin β4: A Multi-Faceted Tissue Repair Stimulating Protein in Heart Injury. Curr Med Chem, 2020. 27(37): p. 6294-6305.
- Smart, N., et al., Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature, 2007. 445(7124): p. 177-82.
- Trenkwalder, T., et al., Thymosin-β4-mediated therapeutic neovascularization: role of the PI3K/AKT pathway. Expert Opin Biol Ther, 2015. 15 Suppl 1: p. S175-85.
- Ehrlich, H.P. and S.W. Hazard, 3rd, Thymosin beta4 enhances repair by organizing connective tissue and preventing the appearance of myofibroblasts. Ann N Y Acad Sci, 2010. 1194: p. 118-24.
- Vukojević, J., et al., Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul Pharmacol, 2018. 106: p. 54-66.
- Guarnera, G., A. DeRosa, and R. Camerini, The effect of thymosin treatment of venous ulcers. Ann N Y Acad Sci, 2010. 1194: p. 207-12.
- Goldstein, A.L., E. Hannappel, G. Sosne, and H.K. Kleinman, Thymosin β4: a multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther, 2012. 12(1): p. 37-51.
Certificate of Analysis (COA)
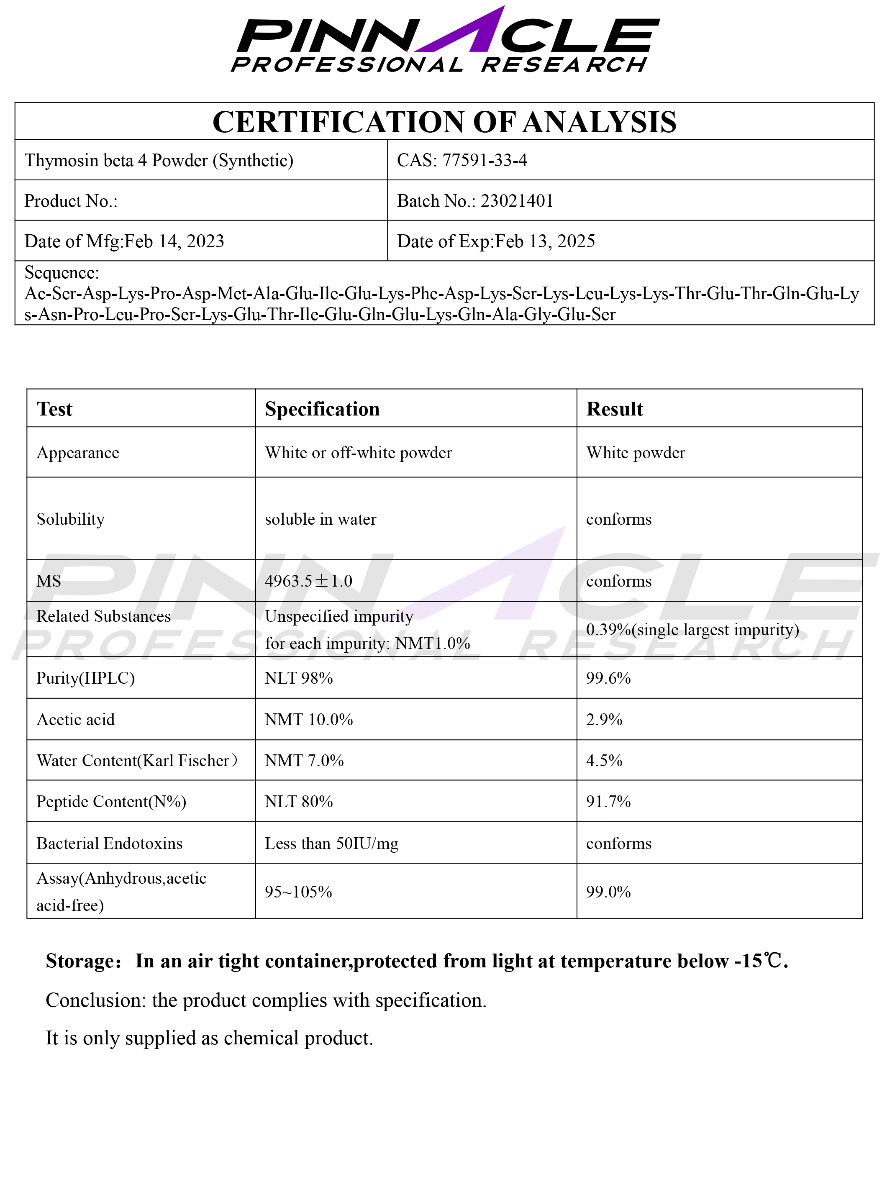
High Performance Liquid Chromatography (HPLC)
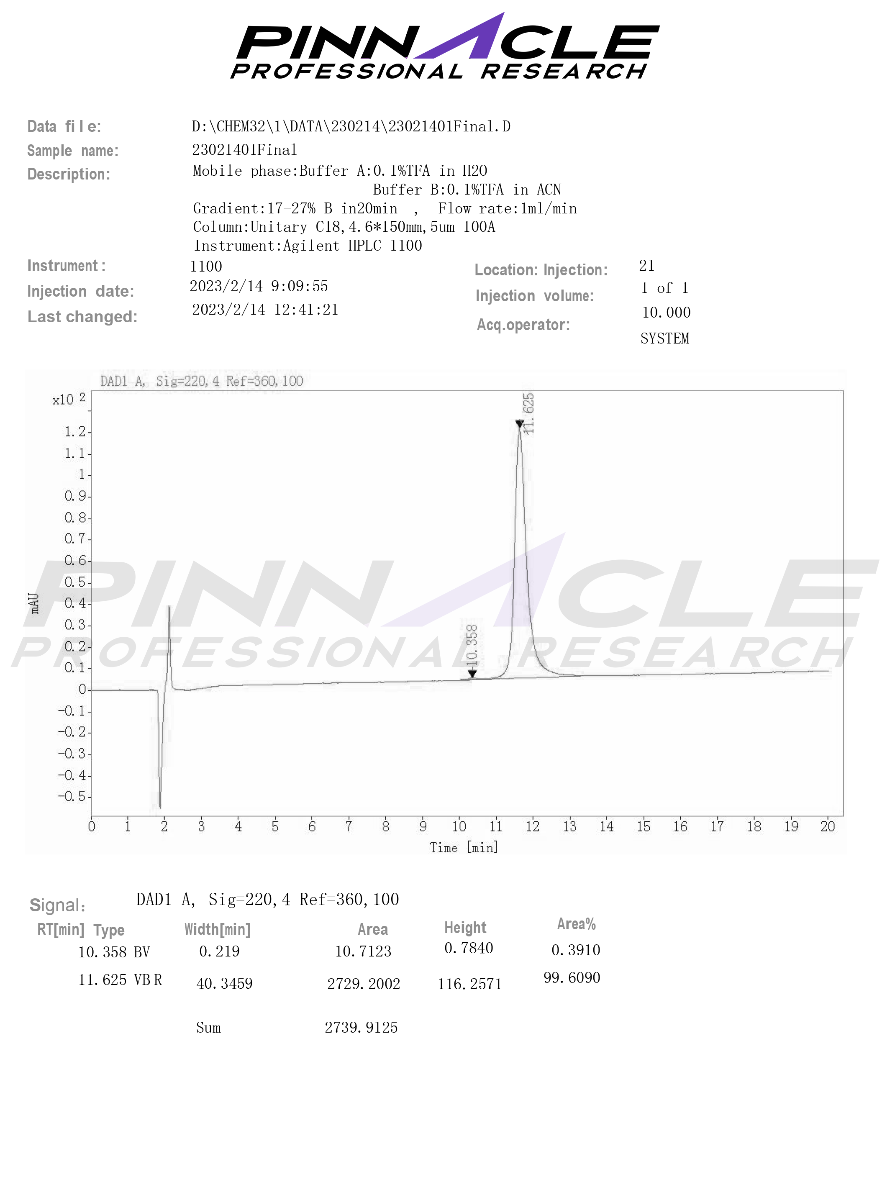
Mass Spectrometry (MS)
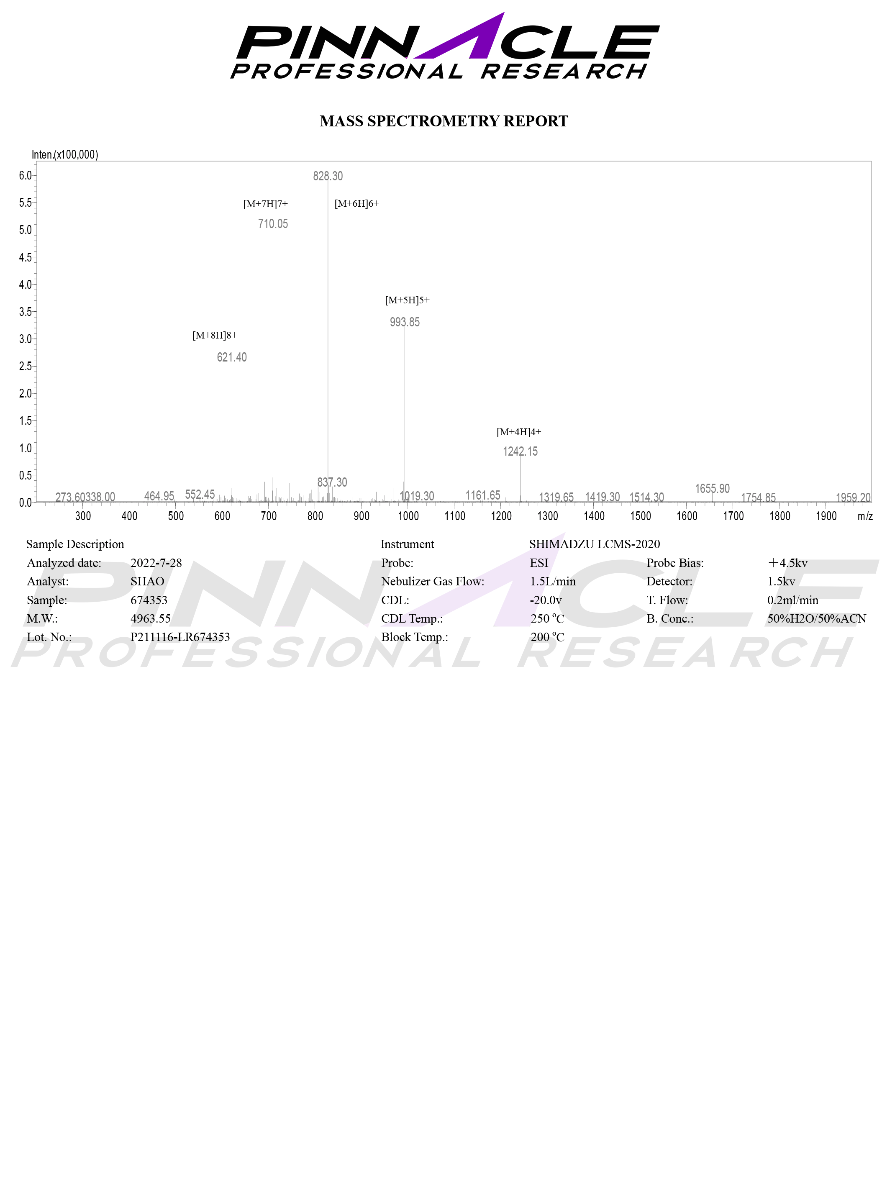
-
Will buy againI ordered a couple of products, they arrived very quickly.
Posted on
-
Clean productsClean products
Posted on
-
Great as alwaysBottles always arrive on or near time. The product themselves is authentic.
Posted on
-
High qualityThis TB-500 5MG is amazing!
Posted on